Obesity management
V-OLET
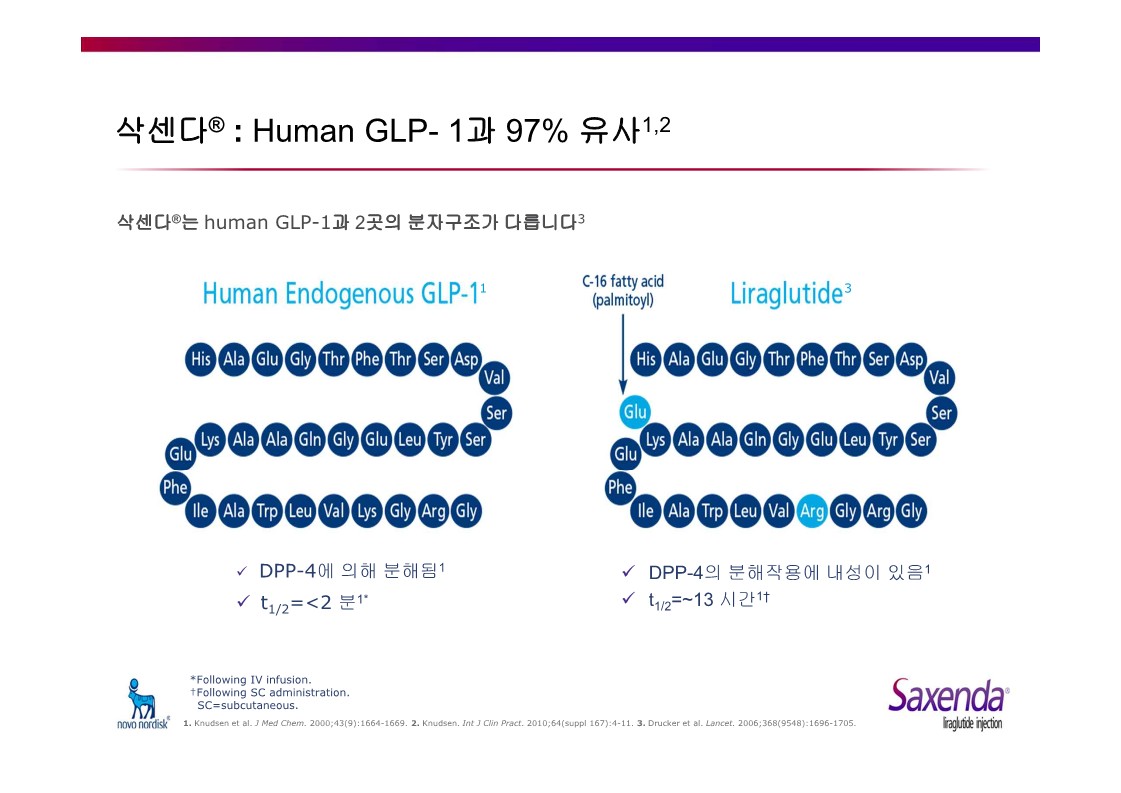
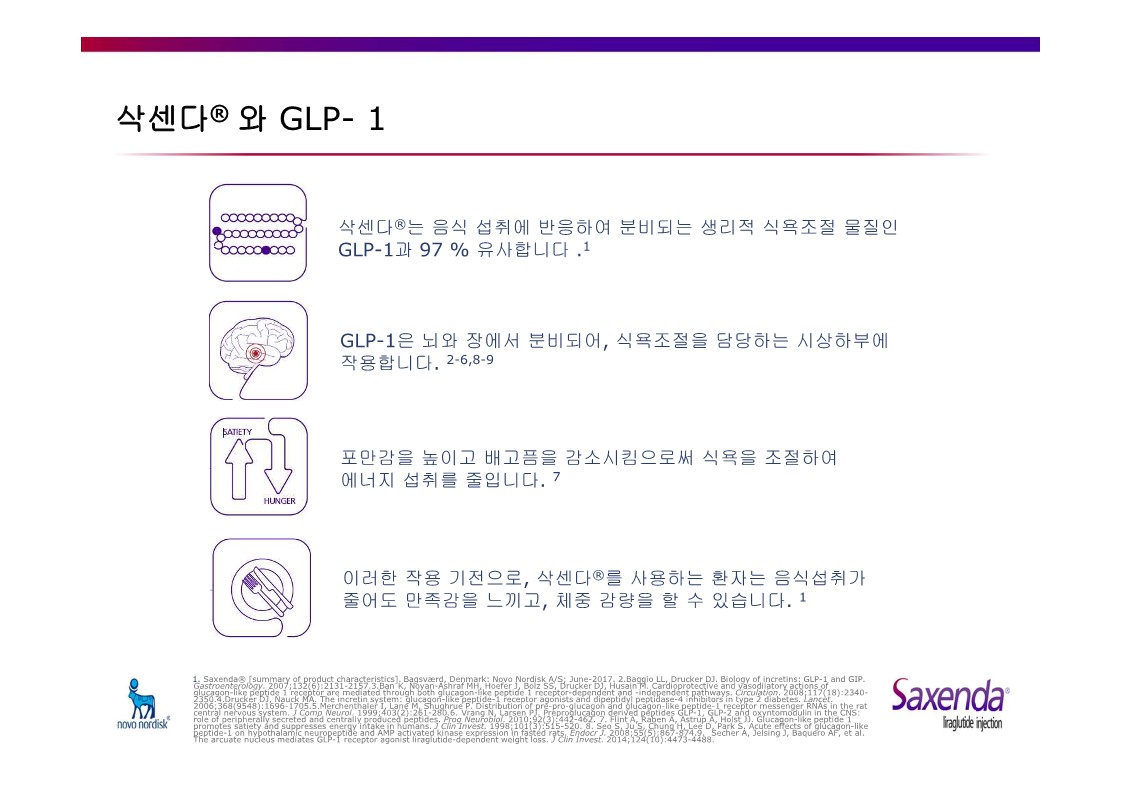
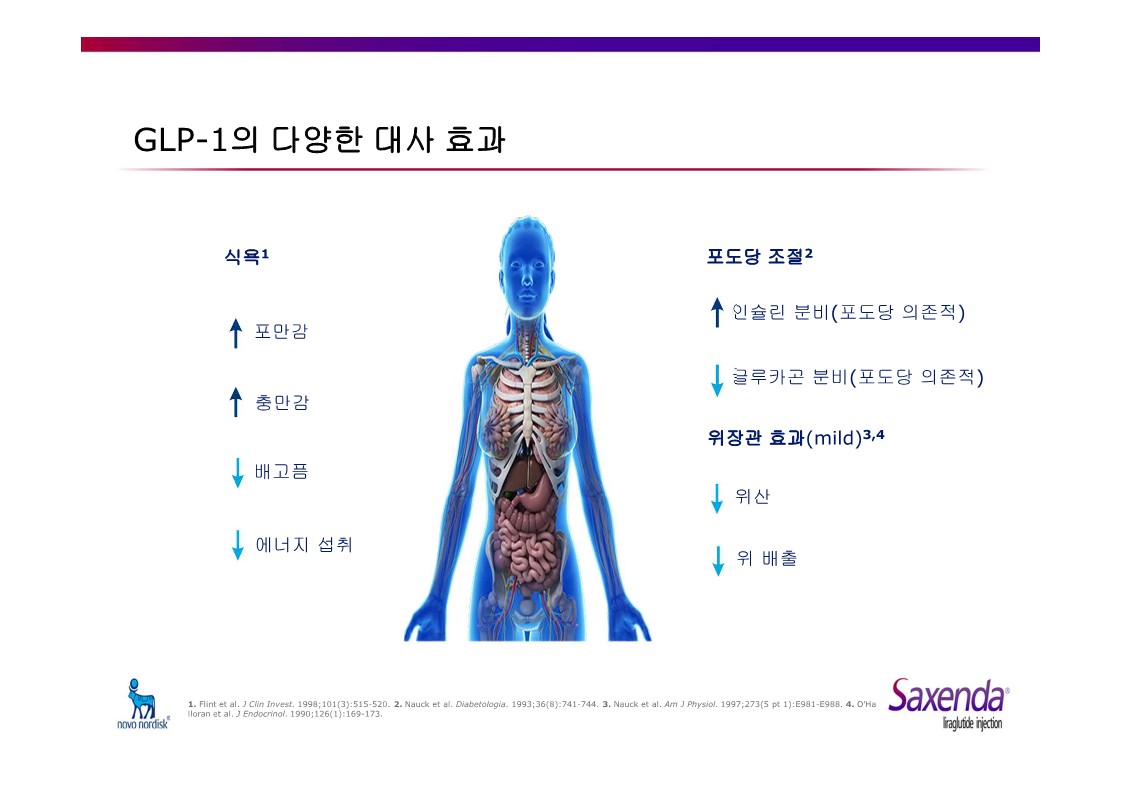
Saxenda
V-OLET
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

V Ollette Dabindo FAQ
– Packaging unit: 2mL x 5vial / BOX
– 2021 price information

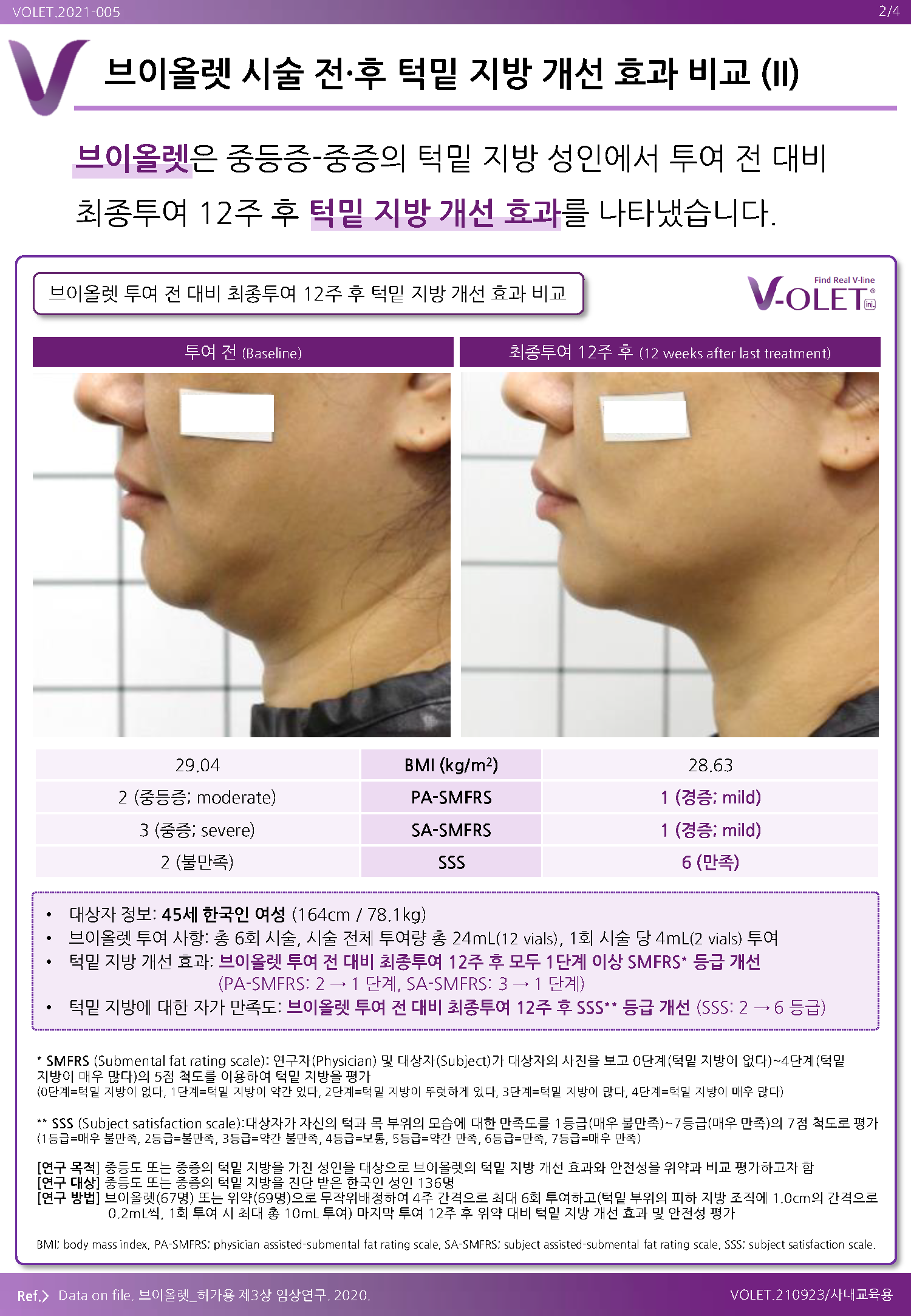
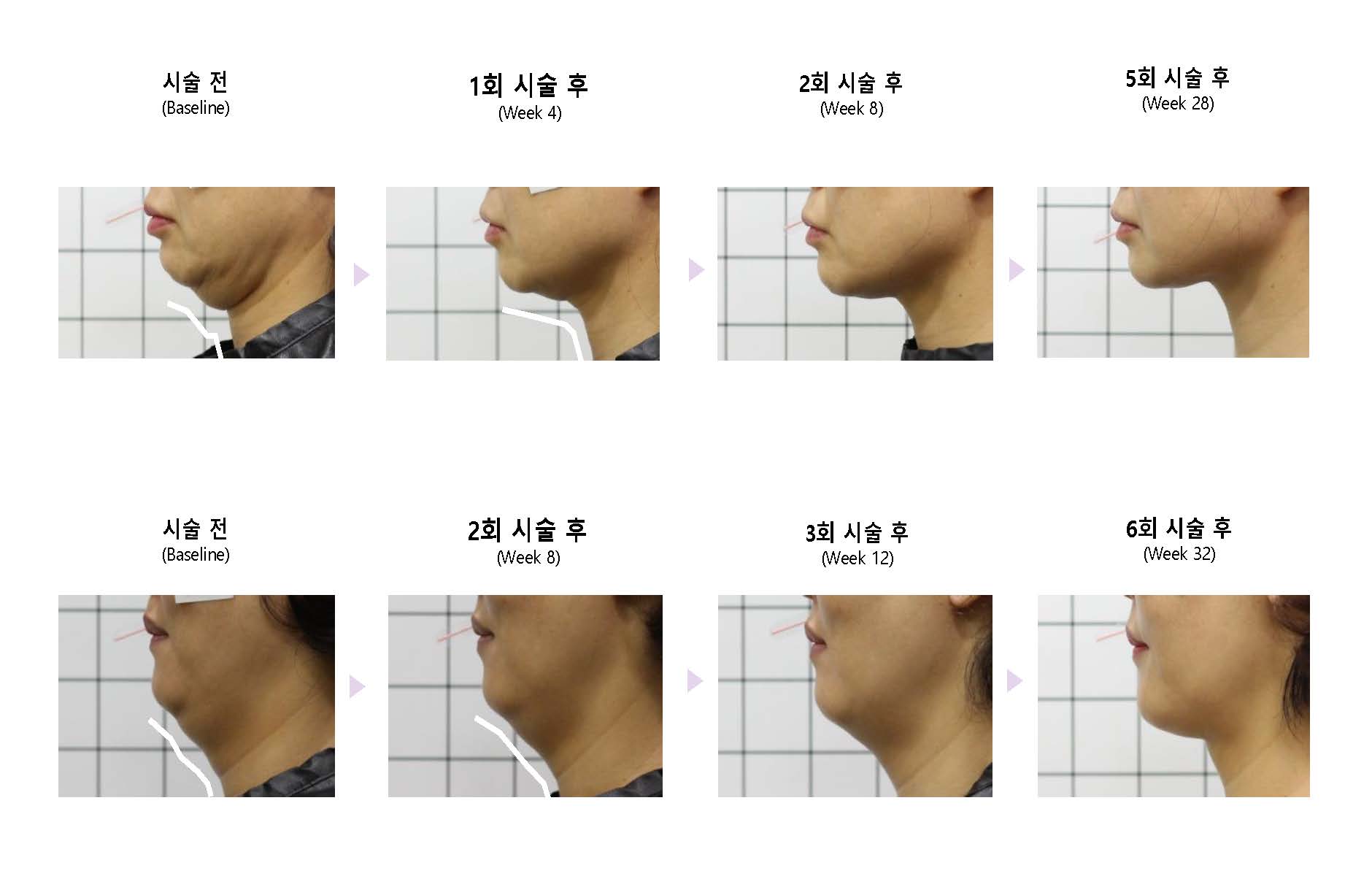
Violet is a submental fat improvement injection and is the only approved specialty drug in Korea.
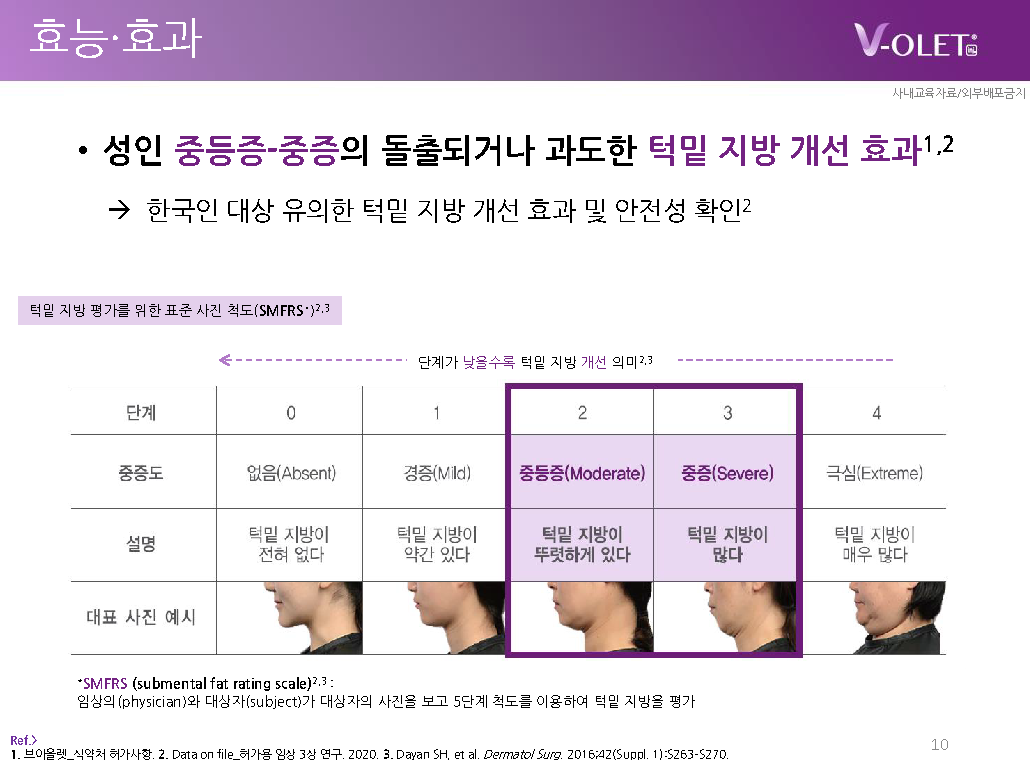
Efficacy/Effectiveness: Improvement of moderate to severe protruding or excessive submental fat in adults.
1. Violet_Ministry of Food and Drug Safety approval.
The main ingredient is deoxycholic acid (DCA), 20mg/2mL per vial.
1. Violet_Ministry of Food and Drug Safety approval.
It is 18 months from the date of manufacture at room temperature (1~30℃).
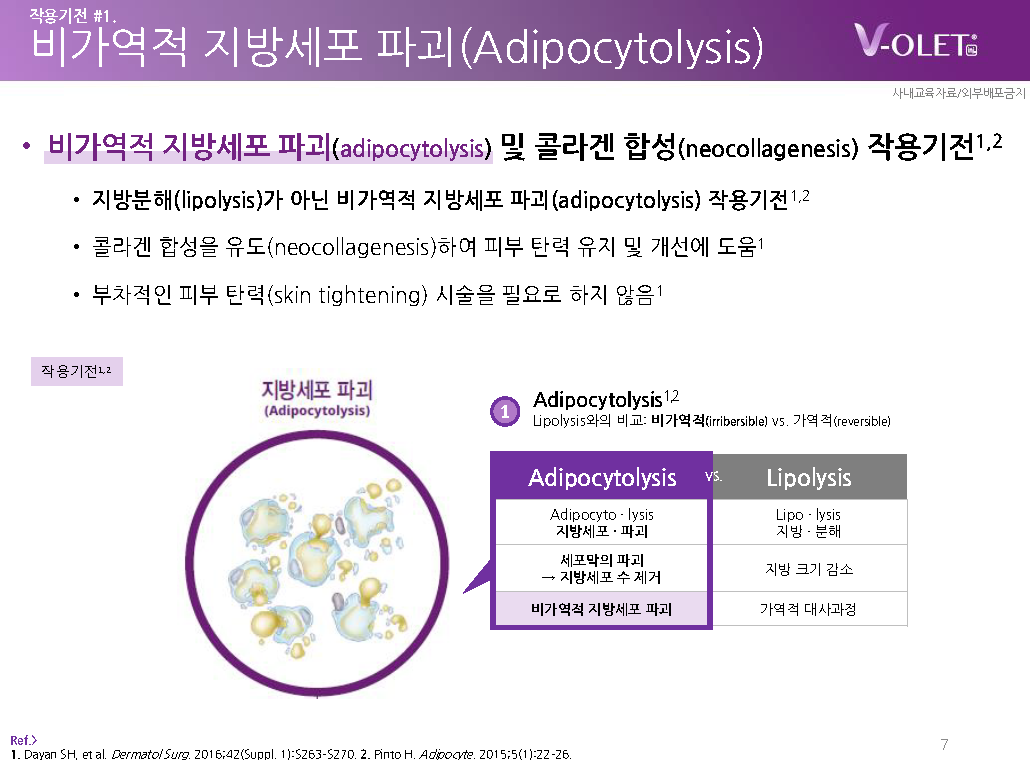
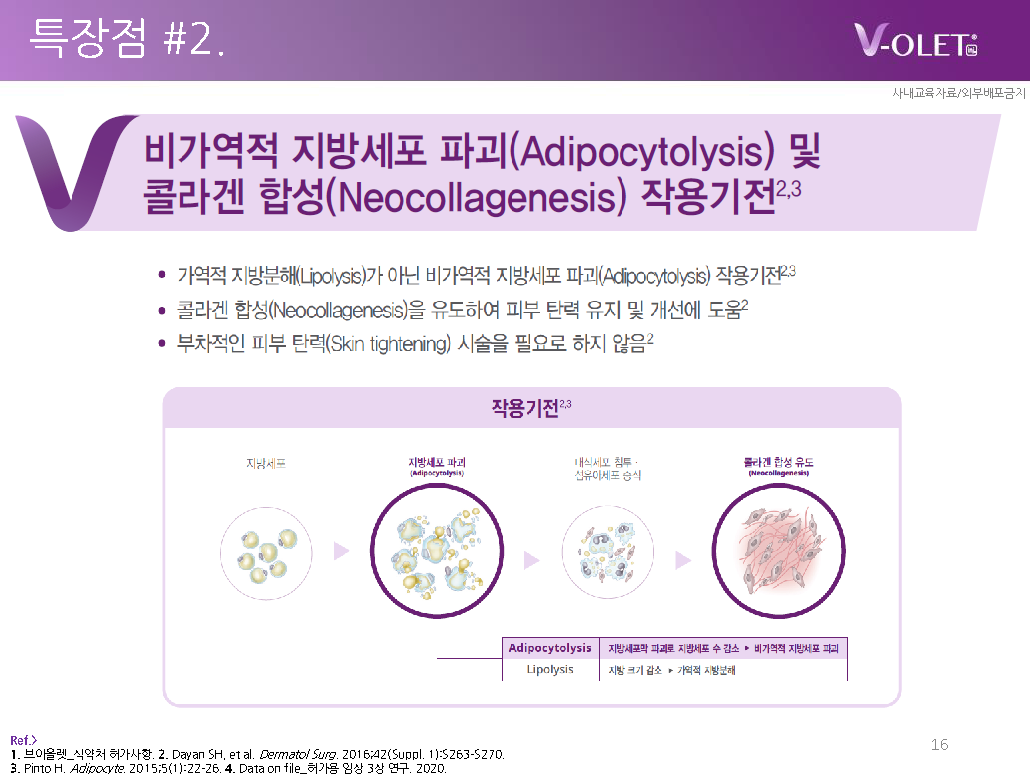
– In the case of simple lipolysis, it represents a reversible metabolic process that temporarily reduces the size of fat.
– In comparison, Violet physically destroys the fat cell membrane and removes the number of adipocytes.
– Indicates the mechanism of action of irreversible fat cell destruction (adipocytolysis).2,3
– Additionally, Violet helps maintain and improve skin elasticity by ② inducing collagen synthesis (neocollagenesis).2
※ Violet can be classified into two main features based on its mechanism of action: ① Irreversible destruction of fat cells (adipocytolysis) ② Induction of collagen synthesis (neocollagenesis)
2. Dayan SH, et al. Dermatol Surg. 2016;42(Suppl. 1):S263–S270. 3. Pinto H. Adipocyte. 2015;5(1):22–26
– Since the mandibular border nerve corresponds to the motor branch of the facial nerve, nerve damage can cause asymmetrical laughter or facial muscle paralysis due to paralysis of the depressor labial muscle. 1,4,5
– Therefore, to avoid possible nerve damage, injections within or very close to the branch of the mandibular border of the facial nerve (1-1.5 cm) should be avoided. 1,4,5
1. Violet_Ministry of Food and Drug Safety approval. 4. Data on file_Phase 3 clinical study for approval. 2020.
5. Shamban AT. Plast Reconstr Surg Glob Open. 2016;4(12 Suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):e1155.
– It is advisable to systematically determine the amount of use, number of treatments, and treatment interval by comprehensively evaluating the patient's SMF grade and level of pain after treatment.
– It is recommended that the number of treatments is determined by the thickness of the SMF and the amount used is determined by the SMF area.
– As a guide, we recommend 2 to 3 treatments at intervals of at least 1 month (1 to 3 months), with an average of 2 vials per treatment.
– According to the experience of doctors who have used DCA injections, it takes at least two treatments to be effective.
– According to the permit, a maximum of 10mL (i.e. 5vials) can be used at a time, and a total of 6 treatments can be performed at intervals of 1 month or more. One
1. Violet_Ministry of Food and Drug Safety approval. 4. Data on file_Phase 3 clinical study for approval. 2020.
– We recommend a needle (30G 13mm) rather than a cannula.
– Considering the diffusion of DCA injection, it is important to maintain the treatment interval (2 mg/cm 2 dose per area) and injection volume (0.2 mL).
Therefore, the cannula may be difficult to control during the actual procedure.
– Additionally, pain and nerve damage during the procedure do not come from needling, but from the DCA component itself. Symptoms occur when the nerves are temporarily damaged while the DCA component causes an inflammatory response, but in this case, they tend to heal naturally over time.
– After pinching the skin of the double chin and securing space in the SQ fat layer, the injection is performed. The injection depth appears to be approximately 0.5~0.8mm (although it varies depending on the person), and the angle seems to be possible with a vertical 30G 13mm needle.
Saxenda
 |
 |
 |
 |
 |
 |
 |
 |
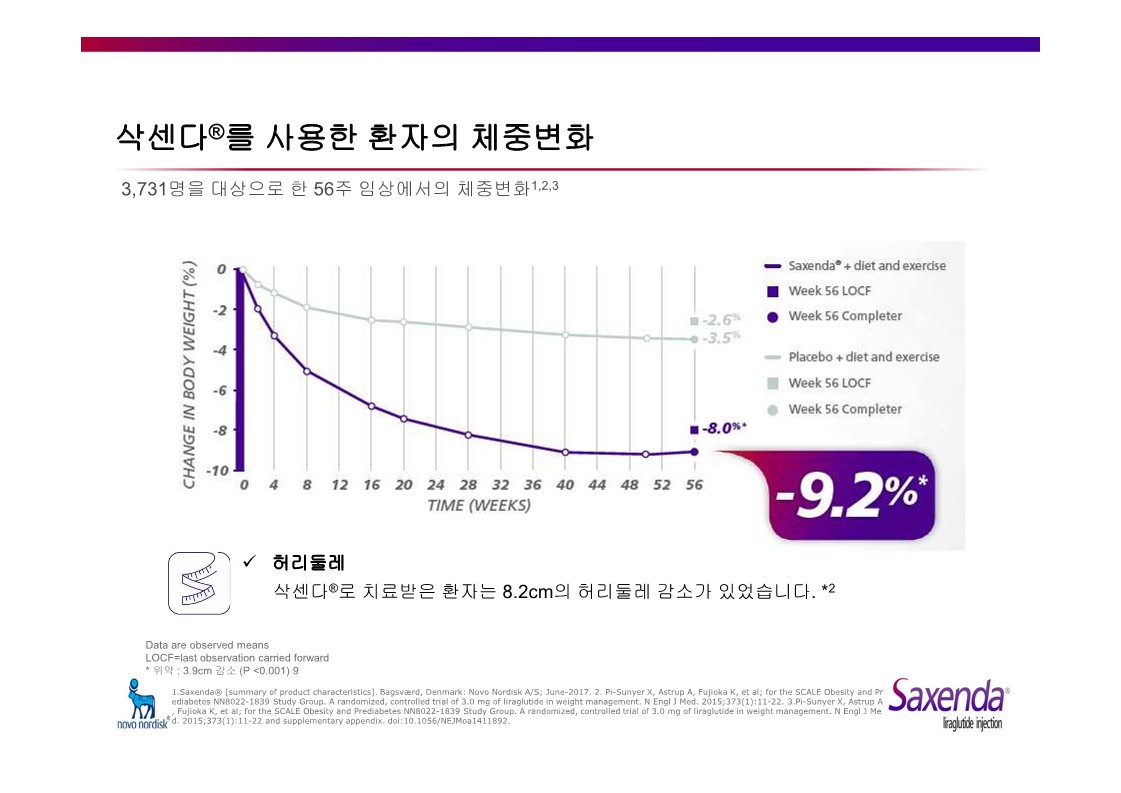 |
 |
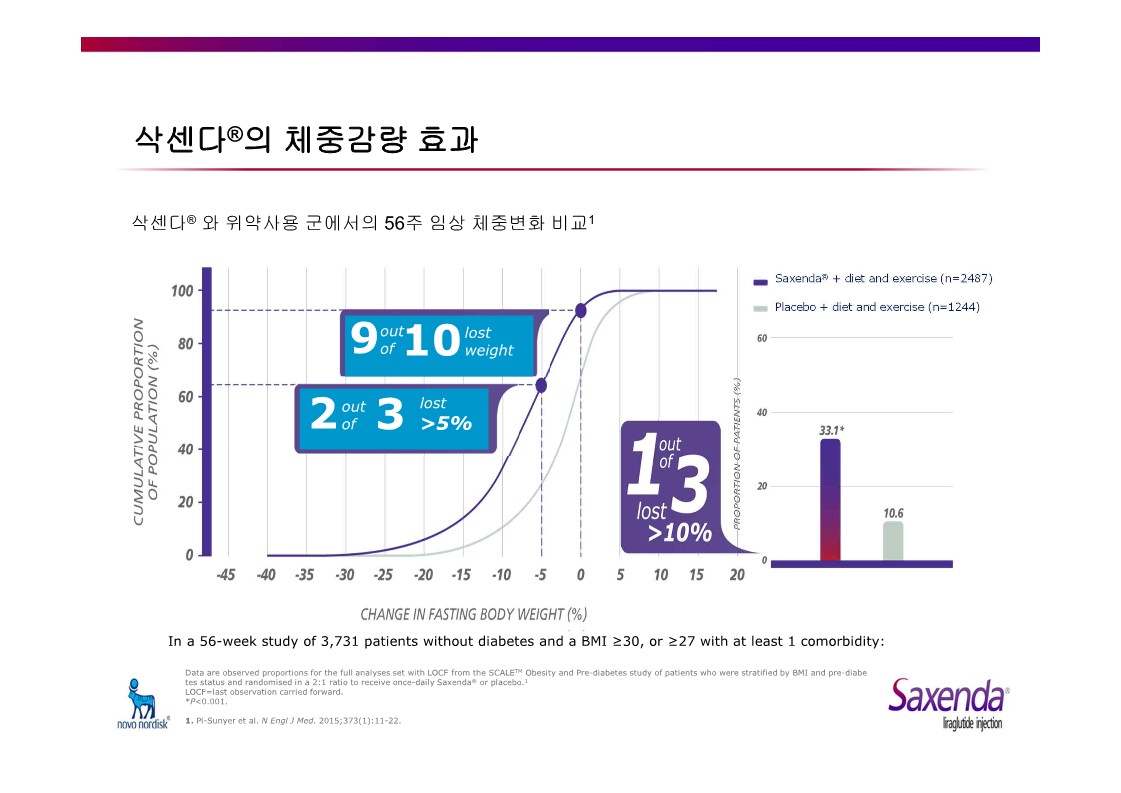 |
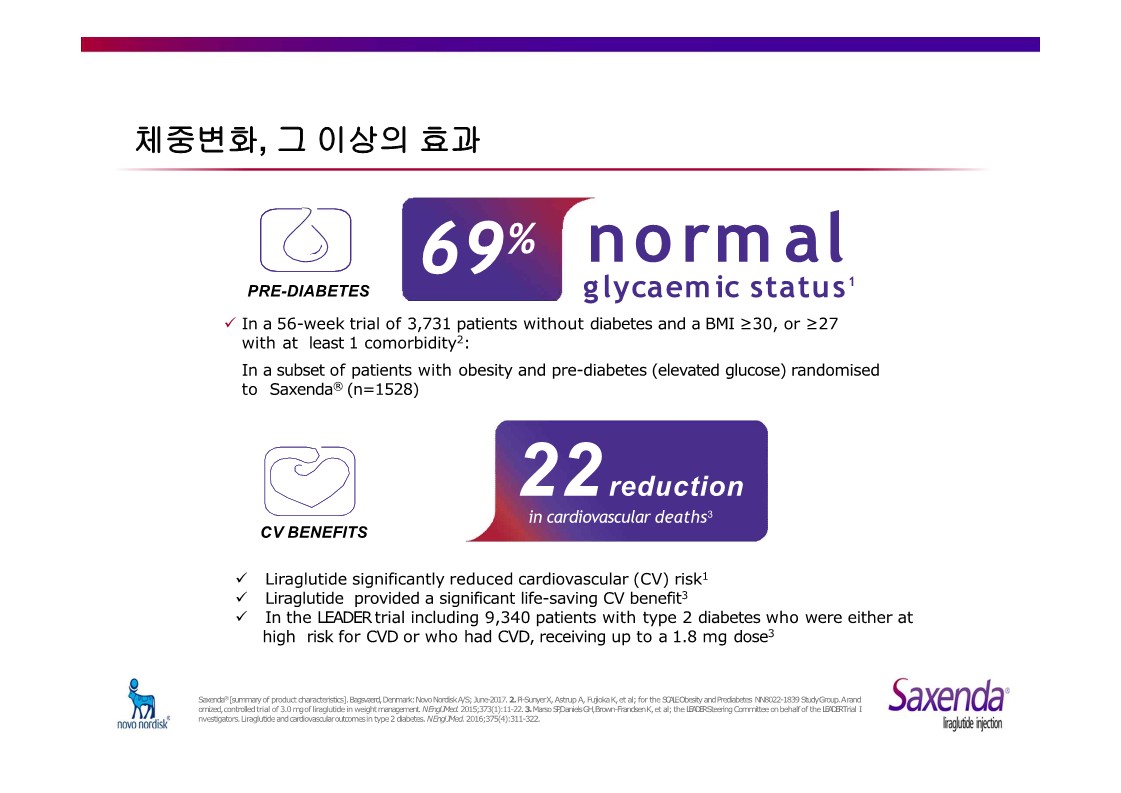 |
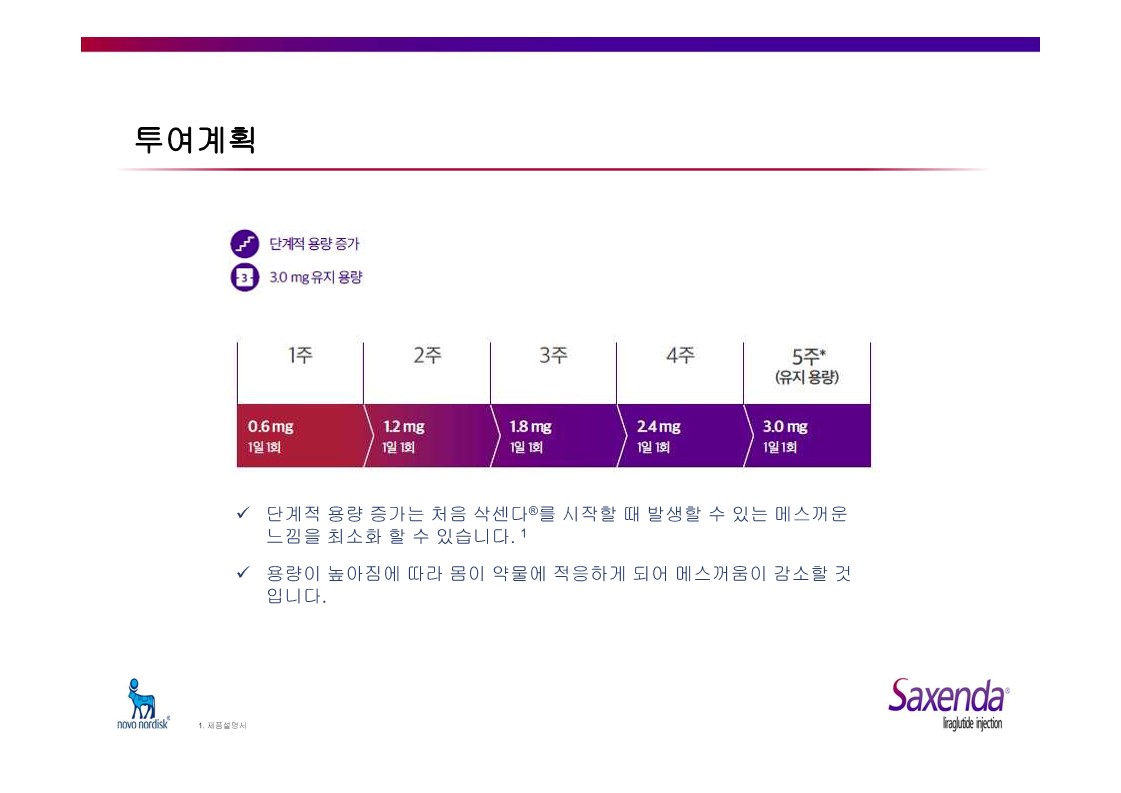 |
 |
 |
 |
 |
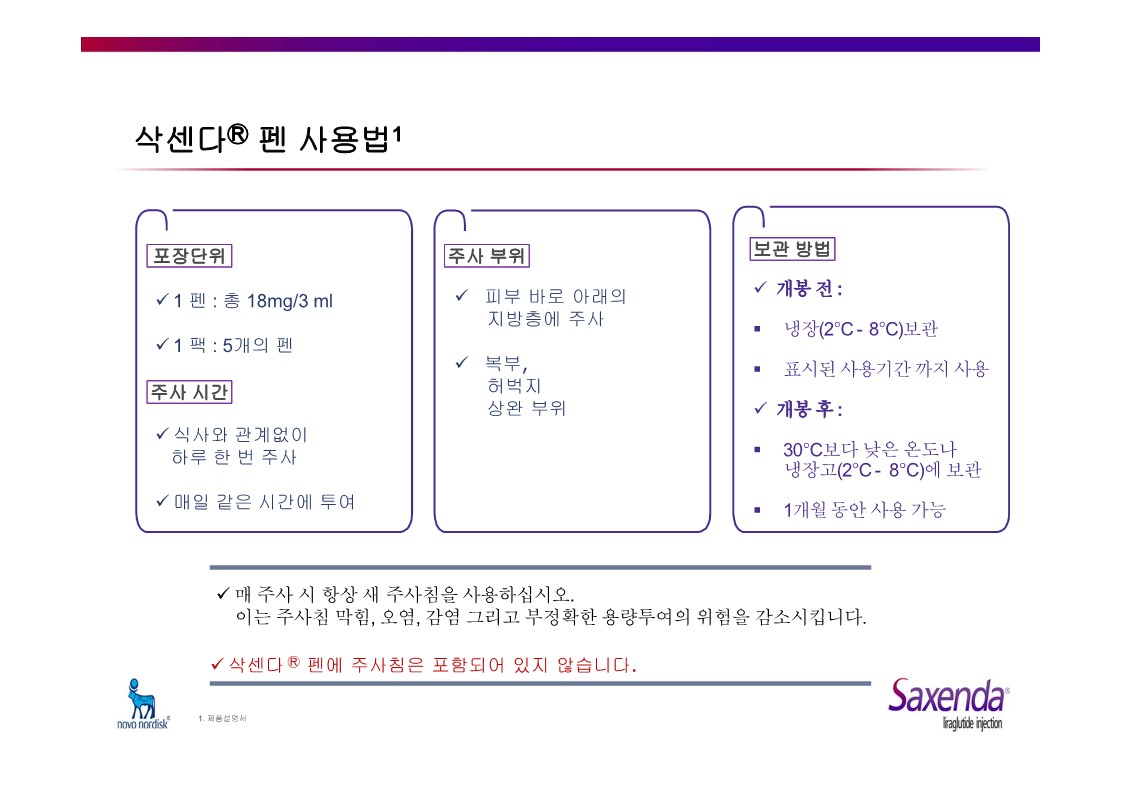 |
 |
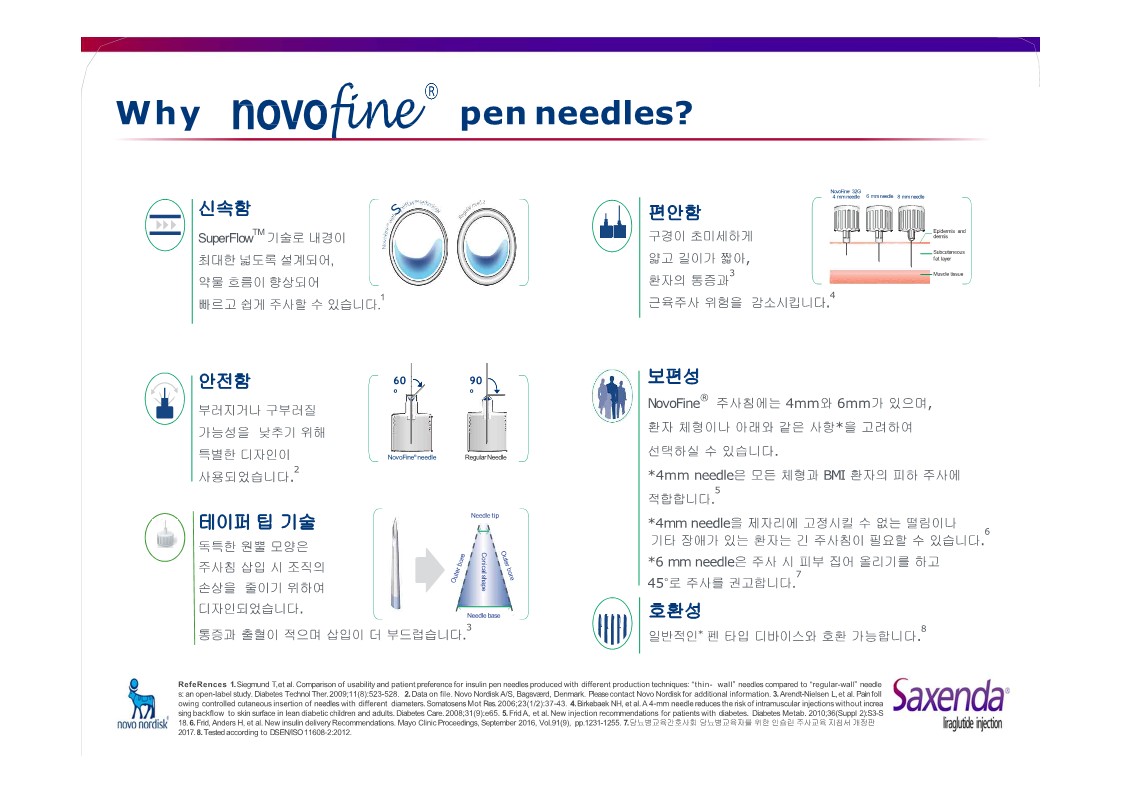 |
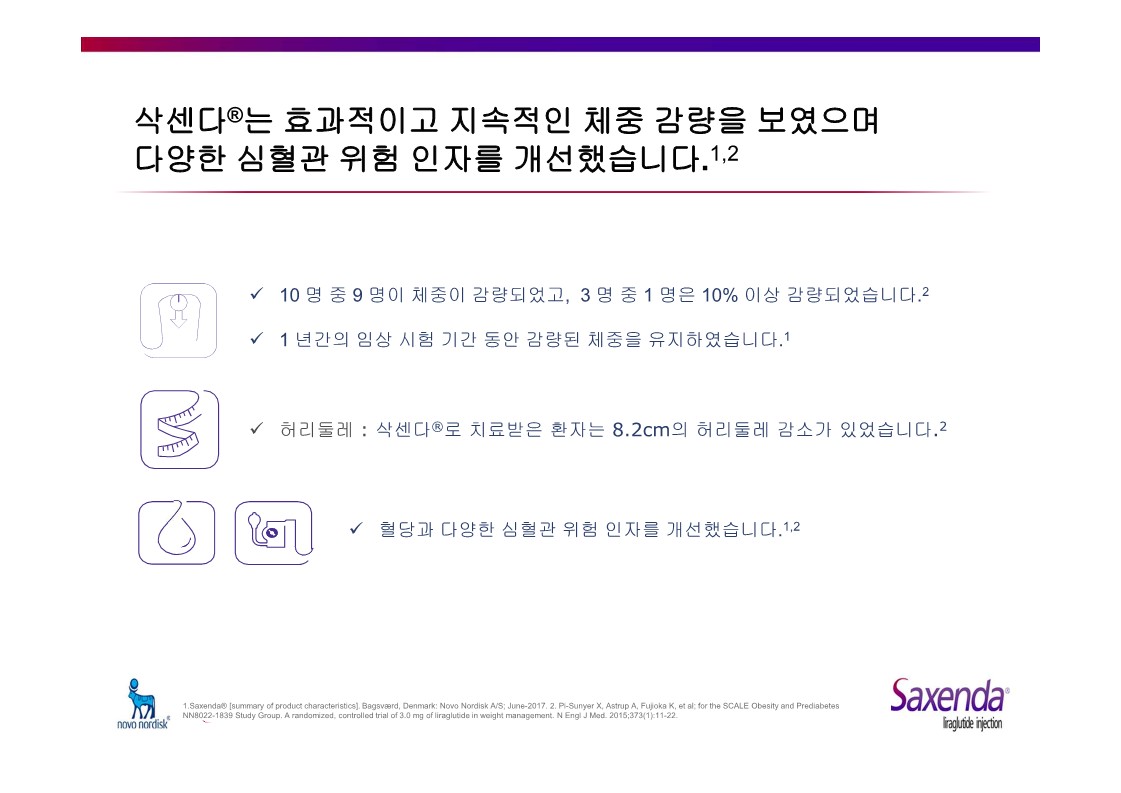 |
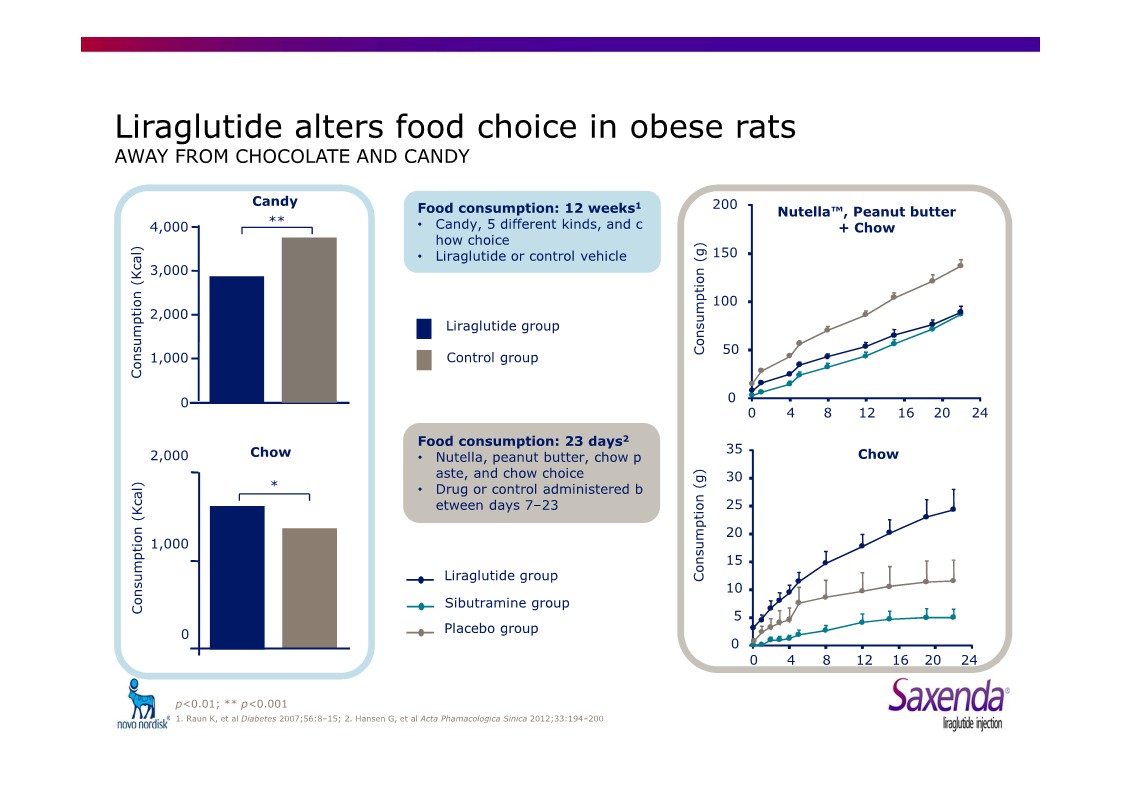 |
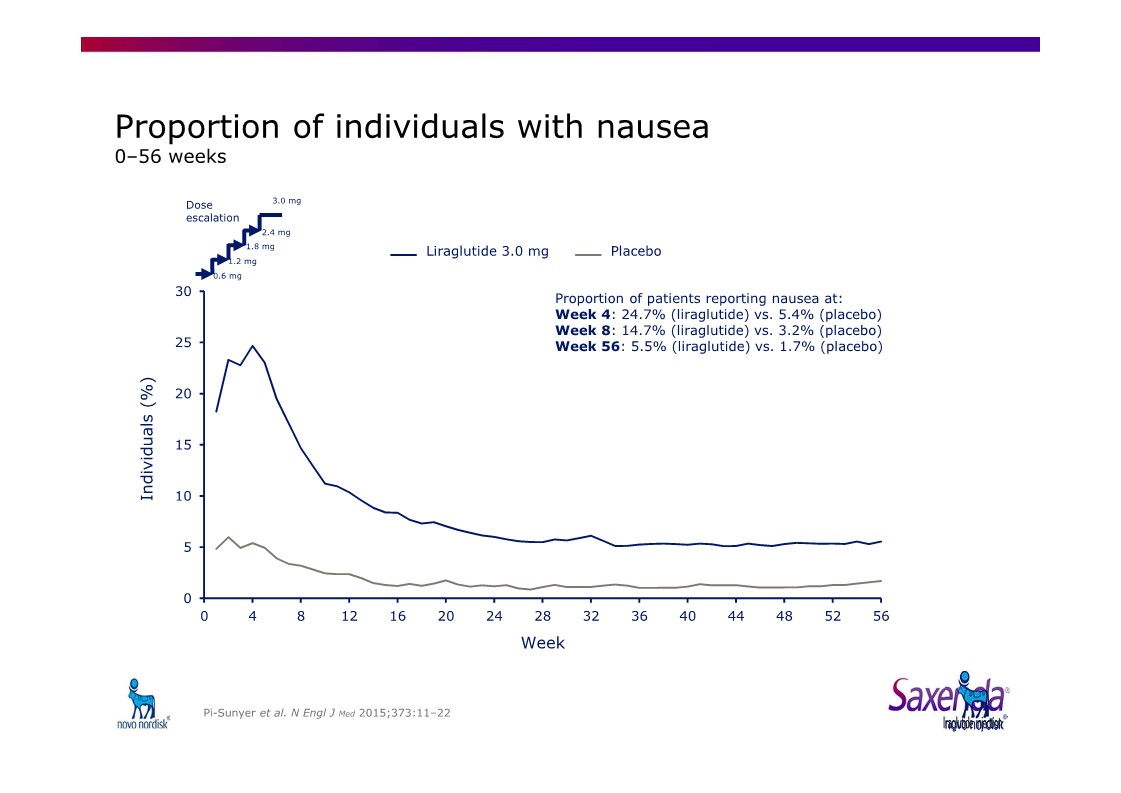 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
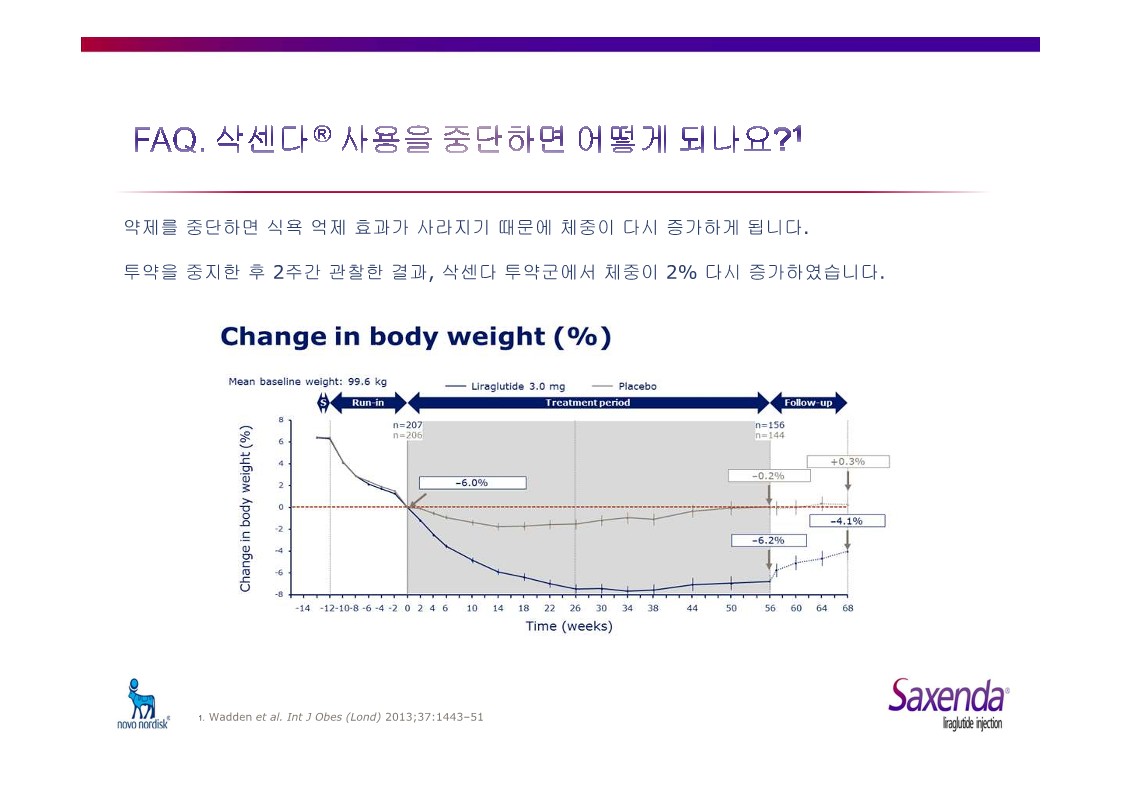 |
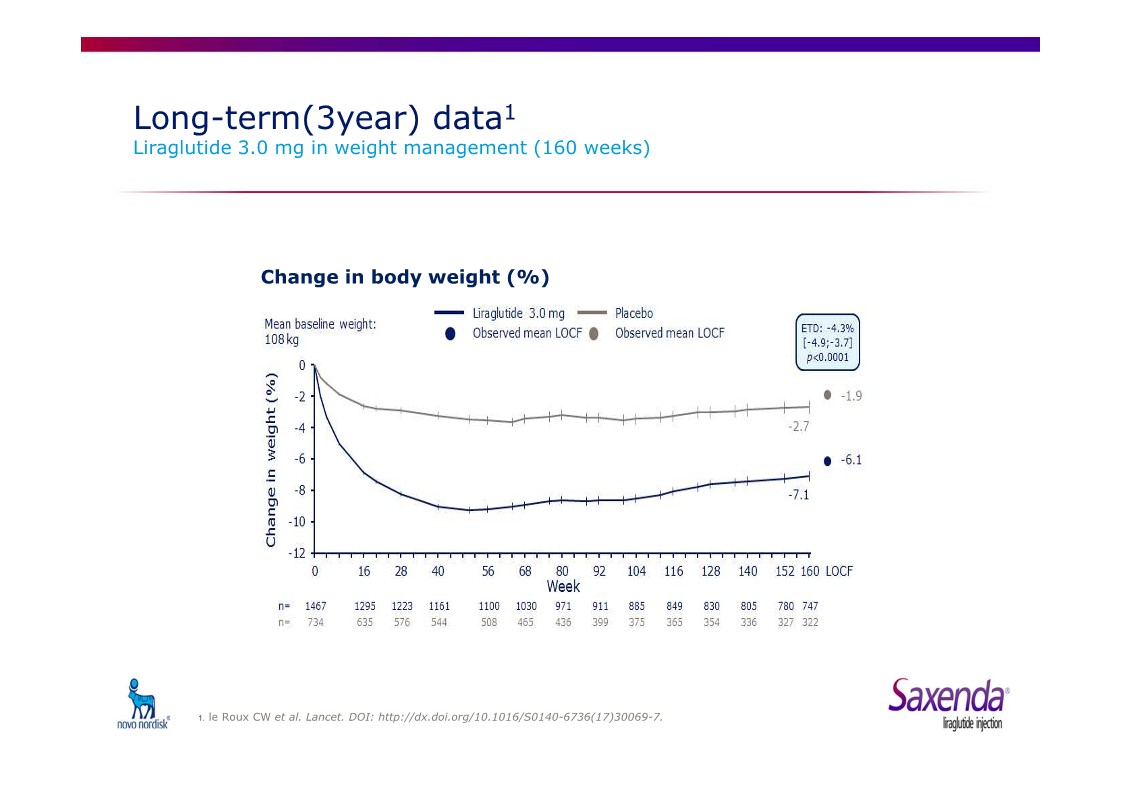 |
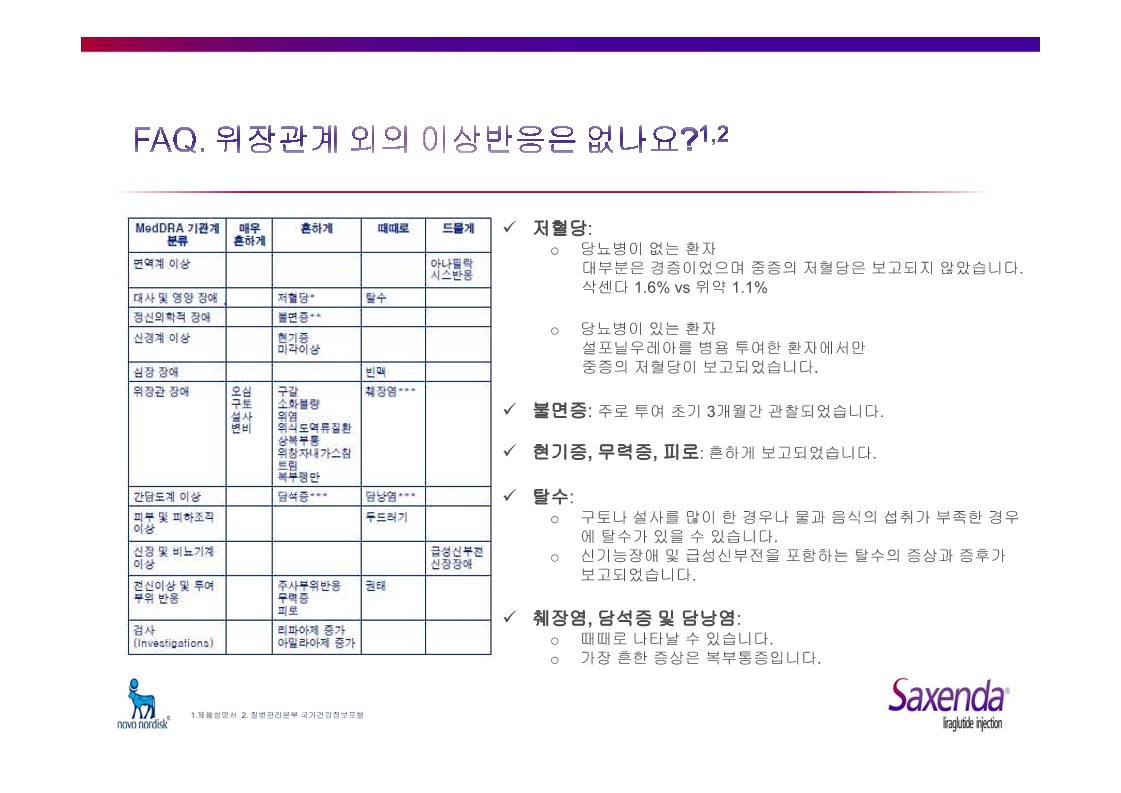 |
 |
 |
 |





